Eczema Severity/Management Drive Multifactorial Disease Burden
Survey study finds no single aspect of disease burden drives overall AD impact
06/29/2022
John McKenna, Associate Editor, BreakingMED™
Vandana G. Abramson, MD, Associate Professor of Medicine, Vanderbilt University Medical Center
A survey of adults with mostly moderate or severe atopic dermatitis (AD) found that most patients rated the negative effect of the condition on their life as being moderate-to-high, with AD severity and spending more time managing AD strongly associated with higher overall disease burden.
Associations between specific disease impact domains—such as sleep, cognitive thinking, and diet—and overall AD impact scores ranged from weak to moderate, with no individual aspect of disease burden correlating strongly with overall impact scores, suggesting that overall AD disease burden is driven by a combination of factors.
For patients with atopic dermatitis (AD), overall disease burden hinges on a wide array of factors, with disease severity and time spent managing the condition greatly influencing patients’ perceived disease burden, according to findings from a survey study.
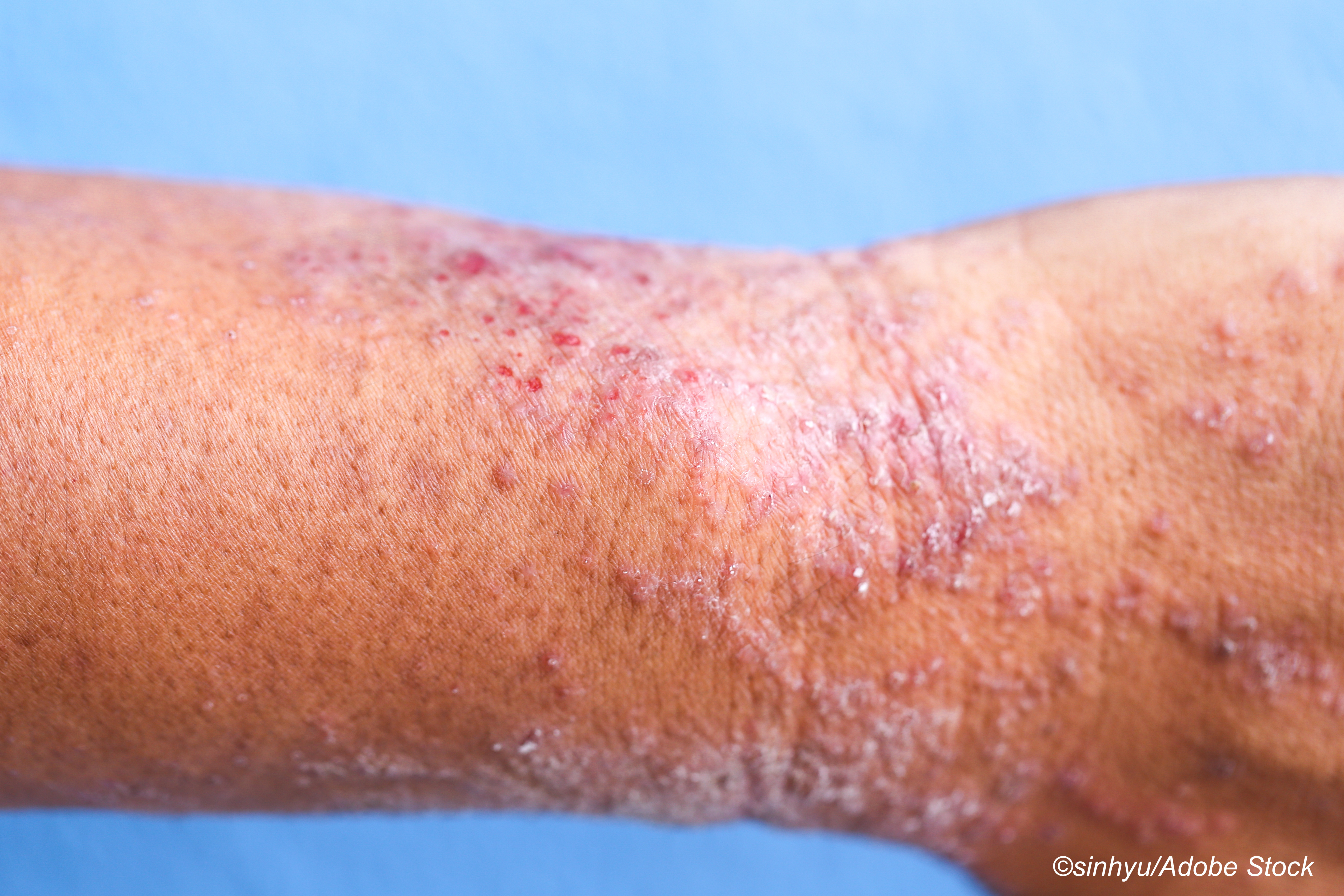
Atopic dermatitis, or eczema, is a relatively common inflammatory skin condition among U.S. adults that is associated with a significant disease burden, study author Aaron M. Drucker, MD, ScM, of the division of dermatology at the Women’s College Hospital of the University of Toronto, and colleagues explained in JAMA Dermatology.
"Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain," they wrote. "Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact."
 In order to better understand the associations between AD and quality of life and treatment preference among patients, Drucker and colleagues conducted an online survey study as part of "an externally led patient-focused drug development (PFDD) meeting" with the FDA in 2019. They used data from adult patients with AD to describe the condition’s association with disease burden, hypothesizing that certain elements of disease burden—impact on sleep, for example—would correlate more strongly with overall disease impact scores than others.
In order to better understand the associations between AD and quality of life and treatment preference among patients, Drucker and colleagues conducted an online survey study as part of "an externally led patient-focused drug development (PFDD) meeting" with the FDA in 2019. They used data from adult patients with AD to describe the condition’s association with disease burden, hypothesizing that certain elements of disease burden—impact on sleep, for example—would correlate more strongly with overall disease impact scores than others.
But the survey results did not bear out this hypothesis, the study authors found.
"In this survey of adults with mostly moderate or severe AD, most rated the negative effect on their life as being moderate-to-high," they wrote. "Atopic dermatitis severity and spending more time managing AD were strongly associated with higher overall disease burden. No single element of disease burden correlated particularly strongly with overall disease burden."
These findings, Drucker and colleagues concluded, highlight the fact that AD disease burden "is multidimensional and heterogeneous. Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed."
For this analysis, Drucker and colleagues pulled data from the externally led PFDD "More Than Skin Deep" survey. This 32-item electronic survey was administered from Aug. 1, 2019-Oct. 11, 2019, and was disseminated via a number of sources, including patient advocacy groups, social media, and clinicians; thus, the study authors could not calculate a response rate for the survey.
Participants self-identified as having AD by answering the question "Which of the following best describes your (primary) relationship to atopic dermatitis (i.e., eczema)?" with the response "I have eczema." Survey participants were entered for a chance to win a $50 Amazon gift card on completion. Respondents were asked to rate the overall impact of their AD in the past month, as well as to rate specific elements of disease burden on an ordinal scale, ranging from 1 (no impact) to 5 (significant impact). Patients were also asked to about "current mood and mood changes at the worst points of AD on a 4-point ordinal scale (not present, mild, moderate, severe)."
Participants rated the impact of AD on the following aspects of disease burden:
- Sleep.
- Cognitive thinking.
- Diet.
- Physical activity.
- Confidence/identity.
- Intimacy.
- Work/education.
- Family activities.
- Leisure activities.
- Stress/resilience.
- Social relationships.
- Life decisions.
- Family dynamics.
A total of 1,065 patients responded to the survey, of whom 11% were ages 18-24, 22% were ages 25-34, 23% were ages 35-50, 27% were ages 51-64, and 17% were age 65 or older. Most respondents were women (83%), and the majority reported either moderate or severe AD (45% and 28%, respectively); eighty percent reported having severe AD when their skin condition was at its worst.
"When asked about the overall disease burden of AD symptoms in the past month, 32 (3%) of respondents reported no impact, 194 (18%) reported a low impact score, 295 (28%) reported a moderate impact score, 228 (21%) reported a high impact score, whereas 316 (30%) reported a significant impact on life," Drucker and colleagues reported. "In the multivariable proportional odds model, current AD severity was strongly associated with higher overall impact scores; moderate AD (odds ratio [OR], 4.13; 95% CI, 2.94-5.79) and severe AD (OR, 13.63; 95% CI, 8.65-21.5) were both associated with greater disease burden compared with mild AD. Spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (11-20 hours spent managing AD: OR, 2.67; 95% CI, 1.77-4.03 versus ≥21 hours spent managing AD: OR, 5.34; 95% CI, 3.22-8.85)."
Importantly, they found that correlations between specific disease impact domains and overall AD impact scores "ranged from weak to moderate (ρ=0.32-0.50), with no individual aspect of disease burden correlating strongly with overall impact scores. Similar results were seen after stratifying the analysis by age, current severity, and time spent managing AD."
Most participants reported mood changes, they added: "424 (40%) reported mild mood changes, 316 (30%) moderate, and 95 (9%) severe. The variable most strongly associated with current mood changes was current AD severity (severe AD: OR, 5.29; 95% CI, 3.41-8.20)."
The fact that no one aspect of disease burden was singled out as being highly associated with overall AD disease burden suggests that AD burden "is driven by a combination of factors," Drucker and colleagues wrote. Moreover, the substantial disease burden linked to time spent managing AD highlights the need for more effective therapies to improve symptoms and reduce the time needed to manage the condition.
Study limitations include that the cross-sectional survey data may not reflect the overall, long-term experience of patients with AD and may be susceptible to recall bias; the study may be subject to selection bias, as it was limited to patients willing to use technology, and those who perceive their AD as more severe or burdensome may have been more inclined to participate than others; and the study "did not use the patient-oriented outcome measure or dermatology life quality index, validated patient-reported outcome measures that could have provided additional insights."
Disclosures
Drucker reported grants from the National Eczema Association (NEA) during the conduct of the study, as well as compensation from the British Journal of Dermatology (reviewer and Section Editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer).
Study coauthor Dainty reported grants from NEA during conduct of the study. Coauthor Begolka reported sponsorships from AbbVie, LEO Pharma, and Eli Lilly paid to NEA, grants from Pfizer paid to NEA, and sponsorship from Sanofi/Regeneron paid to NEA during the conduct of the study; grants from Pfizer paid to institution, honoraria from Pfizer; paid to NEA, honoraria from Incyte; paid to NEA, and salaried work from NEA outside the study. Coauthor Butler reported grants from Pfizer, sponsorships from Sanofi, AbbVie, Eli Lilly, Incyte, LEO, Dermavant, Arena, Galderma, and salaried work from NEA outside the study. Coauthor Capozza reported grants from NEA subcontract during the conduct of the study; grants from LEO received by Global Parents for Eczema Research, grants from Sanofi Genzyme and Regeneron received by Global Parents for Eczema Research, grants from Eli Lilly received by Global Parents for Eczema Research, grants from Incyte Received by Global Parents for Eczema Research, and grants from Galderma Received by Global Parents for Eczema Research outside the study. Coauthor Eftekhari reported grants from NEA during the conduct of the study; grants from Pfizer, Incyte, Sanofi Genzyme and Regeneron, Novartis, Leo Pharma, Boehringer Ingelheim, AbbVie, and Dermira/Lilly outside the study. Coauthor Tullos reported grants from NEA NEA and was the fiscal agent who obtained sponsorship and distributed funds during the conduct of the study.
Sources
Drucker AM, et al "The multidimensional burden of atopic dermatitis among adults: Results from a large national survey" JAMA Dermatol 2022; DOI: 10.1001/jamadermatol.2022.1906.