Clinician Panel Offers Consensus-Based Guidance for Managing Opioid Tx in Advanced Ca Patients
Experts weigh in on three clinical scenarios
06/30/2022
John McKenna, Associate Editor, BreakingMED™
Anupama Brixey, MD, Assistant Professor in Cardiothoracic Imaging, Oregon Health and Science University
Researchers conducted a qualitative analysis in which they used a modified Delphi panel approach to develop consensus-based guidance for the use of certain opioid management strategies for patients with advanced cancer-related pain.
Note that these findings are not guidelines, but rather guidance that highlights areas of consensus and controversy.
While there are reams of information available for the management of opioid use disorder among the general population, little is known about the best approach for managing opioid treatment for patients with advanced cancer—but a new set of consensus-based guidance, published in JAMA Oncology, may offer some insight.
"Regardless of the pain’s etiology, cancer pain management often relies on opioids," study author Katie Fitzgerald Jones, MSN, of the William F. Cornell School of Nursing at Boston College in Chestnut Hill, Massachusetts, and colleagues explained.
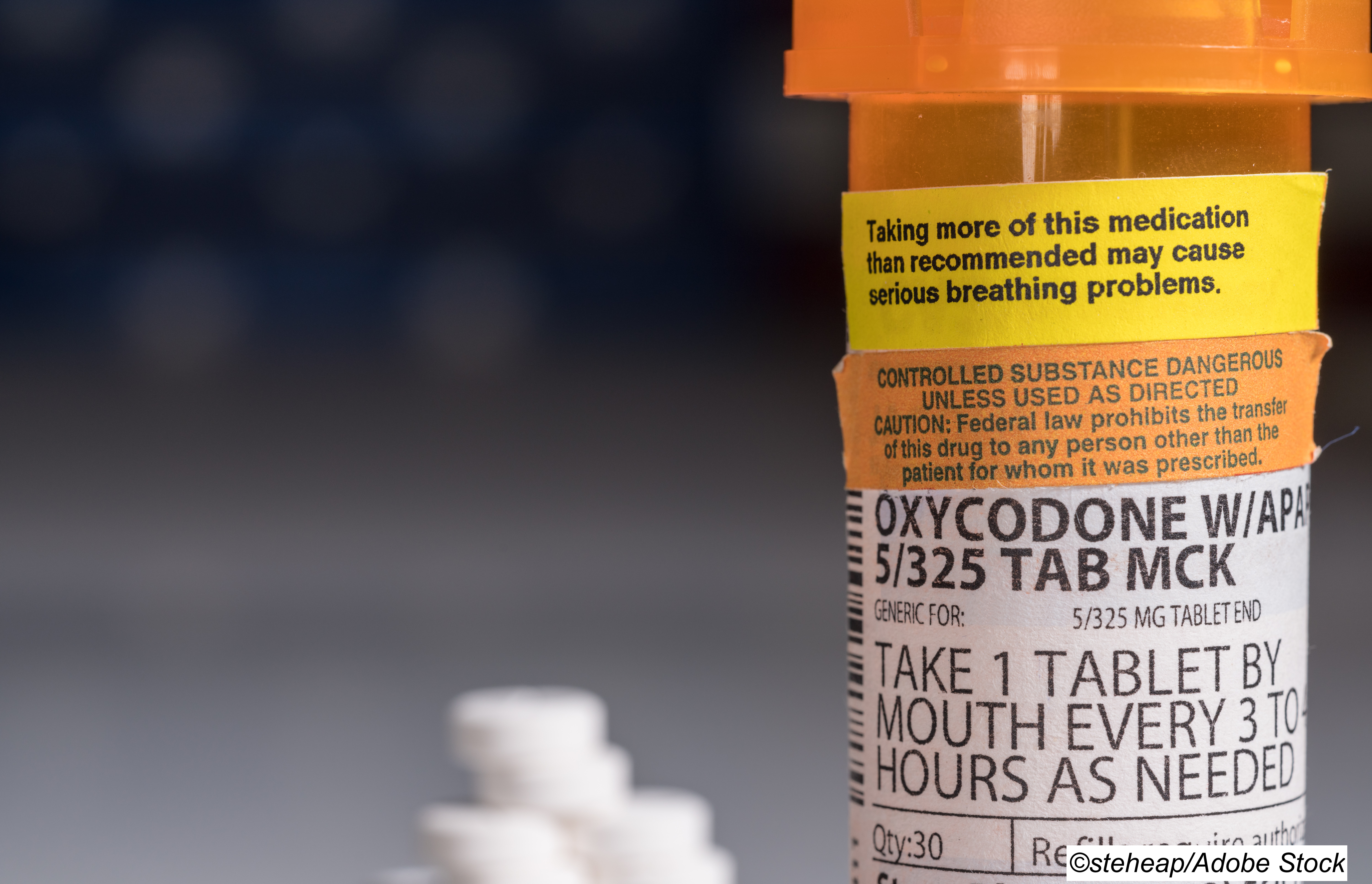 In fact, more than half of people with advanced cancer are prescribed opioids, and guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) call drugs such as morphine and oxycodone "the ’foundation of cancer pain management’ for moderate-to-severe acute pain or pain from metastatic disease."
In fact, more than half of people with advanced cancer are prescribed opioids, and guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) call drugs such as morphine and oxycodone "the ’foundation of cancer pain management’ for moderate-to-severe acute pain or pain from metastatic disease."
But it is also known that cancer patients have high rates of substance use disorder that can lead to overdose and death—and, even without pre-existing opioid use disorder (OUD), many will develop behaviors of opioid misuse, "such as taking more opioids than prescribed or using nonprescribed medications that increase opioid-related risks (e.g., benzodiazepines)."
To better inform treatment decisions for this subset of patients, Fitzgerald Jones and colleagues conducted a qualitative analysis in which they used a modified Delphi panel approach to develop consensus on the appropriateness of certain opioid management strategies commonly used in the general population for patients with advanced cancer-related pain.
For their analysis, the study authors conducted two online modified Delphi panels in order to capture clinical perspectives on three common clinical scenarios related to opioid misuse/OUD and cancer-related pain management. The first (Panel A) focused on scenarios involving a patient with a prognosis of weeks to months, while the second (Panel B) focused on a patient with a prognosis of months to years.
Each panel weighed in on three scenarios, or cases, all of which involved a 50-year-old patient with advanced cancer undergoing active treatment who had cancer-related pain and associated opioid misuse/OUD:
- Case 1: The patient had a recent history of OUD but was not currently on medication for OUD.
- Case 2: The patient had no history of OUD, was taking more opioids than prescribed, and had a negative urine test for the prescribed opioids.
- Case 3: The patient had no history of OUD and was prescribed traditional opioids for pain, then was found to have a positive urine test for the prescribed opioid as well as an unprescribed benzodiazepine.
In all cases, participants were instructed to assume that they had received a waiver from the DEA to prescribe buprenorphine/naloxone, and that it was covered by insurance.
The study authors sent invitations to a range of clinicians with expertise in palliative care and addiction. More than 60 were invited to participate in each panel. In panel round 1 (Aug. 10-25, 2020), "participants reviewed, rated, and commented on the 3 clinical scenarios," as well as management strategies including adjusting opioids, increasing monitoring, tapering opioids, transitioning to buprenorphine/ naloxone, or recommending methadone. "Participants used a 9-point Likert scale to rate the appropriateness of each strategy, where 1 = ’very inappropriate’ and 9 = ’very appropriate,’ and were asked to explain their responses in free-text boxes," they explained.
In round 2 (Sept. 10-17, 2020), participants reviewed bar charts showing participant responses; color-coded statements described whether an agreement had been reached among the panel and which strategies were deemed appropriate. Then, participants used anonymous discussion boards to discuss the results of the first round with their fellow panelists.
In round 3 (Sept. 17-Oct. 8, 2020), participants were asked to provide final responses that reflected the dialogue from round 2.
A total of 129 clinicians were invited to participate in the study—120 (93%) participated in at least one round, and 84 (70%) participated in all three rounds. The majority of participants were White (78%), female (62%), and held MD or DO degrees (96%).
"Of the 14 strategies considered across the three scenarios, both panels reached identical decisions on 12 management strategies," the study authors wrote. "Of these 12 strategies, four were deemed appropriate, four inappropriate, and two of uncertain appropriateness by both panels. The appropriateness of two remaining management strategies in case 1 differed according to prognosis."
The results:
- Case 1: Recent history of OUD, not on medication for OUD.
- Regardless of prognosis, it was deemed appropriate to begin buprenorphine/naloxone treatment, as it was considered a reasonable and safe strategy to "control pain and decrease risk of ’reactivating’ OUD."
- Regardless of prognosis, it was deemed inappropriate to send the patient to a methadone clinic, as it was deemed unlikely that a methadone clinic would address cancer pain, and attending the clinic would be burdensome for those with advanced cancer and "not possible" for those with limited prognoses.
- Initiating treatment with a full agonist was deemed of uncertain appropriateness among patients with a shorter prognosis and inappropriate for patients with a prognosis of over 1 year.
- Initiating treatment with methadone dosed 2-3 times per day, prescribed outside of a methadone clinic, was deemed appropriate for those with a shorter prognosis but of uncertain appropriateness in those with a longer prognosis. "Participants discussed that prescribing methadone for OUD is not legal outside of opioid treatment programs but could be appropriate for a dual indication of pain and OUD," they noted.
- Case 2: No history of OUD, taking more opioids than prescribed, and urine negative for prescribed opioids.
- Regardless of prognosis, it was deemed appropriate to increase patient monitoring, for example, with additional visits or shorter prescriptions.
- Regardless of prognosis, it was deemed inappropriate to taper the patient’s opioids due to "associated discomfort."
- Regardless of prognosis, it was of uncertain appropriateness to increase the patient’s opioids or to transition the patient to buprenorphine/naloxone. For the latter, participants "expressed concern that buprenorphine/naloxone may not be sufficient to control pain, and was less appropriate for people without OUD (and many reported that they could not make a diagnosis of OUD in this case)."
- Case 3: No history of OUD and prescribed traditional opioids for pain, then found to have urine drug tests positive for the prescribed opioid and also for a benzodiazepine that was not prescribed.
- Regardless of prognosis, it was deemed appropriate to continue the patient’s opioids and to increase monitoring.
- Regardless of prognosis, it was deemed inappropriate to taper the patient’s opioids or to transition to buprenorphine/naloxone. "Because the pain was well-controlled, experts did not recommend a change and indicated rotation to buprenorphine/ naloxone would not address benzodiazepine use. They also expressed hesitation to rotate to buprenorphine/naloxone without a diagnosis of an OUD but would consider using other [FDA]-approved buprenorphine products."
In an editorial accompanying the study, Joseph Arthur, MD, and Eduardo Bruera, MD, both of the Department of Palliative, Rehabilitation, and Integrative Medicine at the University of Texas MD Anderson Cancer Center in Houston, pointed out that one of the unfortunate side-effects of the measures taken to curb the U.S. opioid epidemic has been that patients with advanced cancer, "such as those described in this study, are now receiving a much lower opioid dose from their clinicians."
Physicians, they explained, have been made to contend with "universal checking of the Prescription Monitoring Program database, ordering and interpreting urine drug screens, frequent need for prior authorization or refusals by insurers, and inadequate stocks of opioids in dispensing pharmacies" as they attempt to care for their patients. As a result, many have lowered their involvement in opioid management and refer these patients to supportive and palliative care instead—meanwhile, advanced cancer patients may be made to suffer their pain for weeks or months as they wait for these referrals to come through.
"Patients such as the ones considered in this important article require considerable time and expertise of an interdisciplinary team," Arthur and Bruera wrote. "It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer. Clinicians aware that help is readily available when patients engage in nonmedical opioid use may be more comfortable prescribing opioids for cancer pain, as was the case 10 years ago. Rapidly accessible teams and resources for nonmedical opioid use management will provide an extra layer of support and contribute to a reduction in burnout related to workload for already heavily burdened oncology teams."
Another consideration for these patients is that, while cancer care continues to evolve, patients continue to receive the same opioid treatments, many of which "are between 80 and 200 years old."
"These rudimentary drugs bind to μ receptors located along the nociceptive pathway, but they also bind to every μ receptor they find, including in the limbic system, thereby starting the reward and antireward pathways that result in nonmedical opioid use or OUD in anywhere from 5% to 20% of those patients exposed to them," they pointed out. "Our main opportunity is to develop much more sophisticated drugs capable of targeting the nociceptive pathway with minimal or no influence outside that area. Unfortunately, one of the major barriers to achieve new ways of relieving pain and suffering in patients with cancer is the lack of support for research in these important aspects of the cancer care."
Limitations of the qualitative study by Fitzgerald Jones et al included a lack of cancer specialists or pharmacists on the modified Delphi panels; participants self-identified their expertise; areas of expertise may have affected the perception of appropriateness for certain strategies; and the management of cancer-related pain and opioid misuse/ OUD "is an area of rapidly changing practice and literature," they added.
"Delphi studies are considered level 5 (expert opinion) evidence," they wrote. "Our findings are not guidelines but rather guidance that highlights areas of consensus and controversy. We acknowledge that optimal strategies may change over time in response to more empirical evidence and experience."
Disclosures
This study was supported by Cambia Health Foundation.
Fitgerald Jones is funded by the National Institute of Nursing Research.
Study coauthor Khodyakov reported grants from Cambia Health Foundation during the conduct of the study; and he is a creator of ExpertLens, an online modified Delphi platform used to conduct expert panels in this study. Coauthor Arnold reported board membership of VitalTalk, editorial payment from AAHPM and UpToDate regarding palliative care topics. Coauthor Bulls reported grants from the National Institutes of Health (NIH) KL2 TR001856 (Rubio) outside the study. Coauthor Ritchie reported grants from NIH, grants from The John A Hartford Foundation, grants from Boye Foundation, consulting fees from AAHPM Consulting, and West Health Institute Consulting, royalties from McGraw Hill, and Wolters Kluwer, and liscensing fees from UCSF outside the study. Coauthor Merlin reported grants from Cambia Health Foundation to institution during the conduct of the study.
Arthur and Bruera had no relevant relationships to disclose.
Sources
Fitzgerald Jones K, et al "Consensus-based guidanxce on opioid management in individuals with advanced cancer-related pain and opioid misuse or use disorder" JAMA Oncol 2022; DOI: 10.1001/jamaoncol.2022.2191.
Arthur J, Bruera E "Managing cancer pain in patients with opioid use disorder or nonmedical opioid use" JAMA Oncol 2022; DOI: 10.1001/jamaoncol.2022.2150.