VANGUARD: Investigational mAb Effectively, Safely Prevented Hereditary Angioedema Attacks
Monthly Tx with garadacimab quickly, consistently reduced number of monthly attacks vs placebo
03/09/2023
Liz Meszaros, Deputy Managing Editor, BreakingMED™
Kevin Rodowicz, DO, Assistant Professor, St. Luke’s University/Temple University
In the VANGUARD trial, monthly treatment with garadacimab in patients with hereditary angioedema significantly reduced hereditary angioedema attacks in patients aged 12 years and older compared with placebo with a favorable safety profile.
Be aware that garadacimab has received orphan-drug designation from the FDA as an investigational treatment for hereditary angioedema.
Once-monthly treatment with subcutaneous garadacimab brought about early and sustained protection from attacks and had a favorable safety profile in patients with hereditary angioedema, according to results from the multicenter, randomized, double-blind, placebo-controlled, phase III VANGUARD trial.
Results are published in The Lancet.
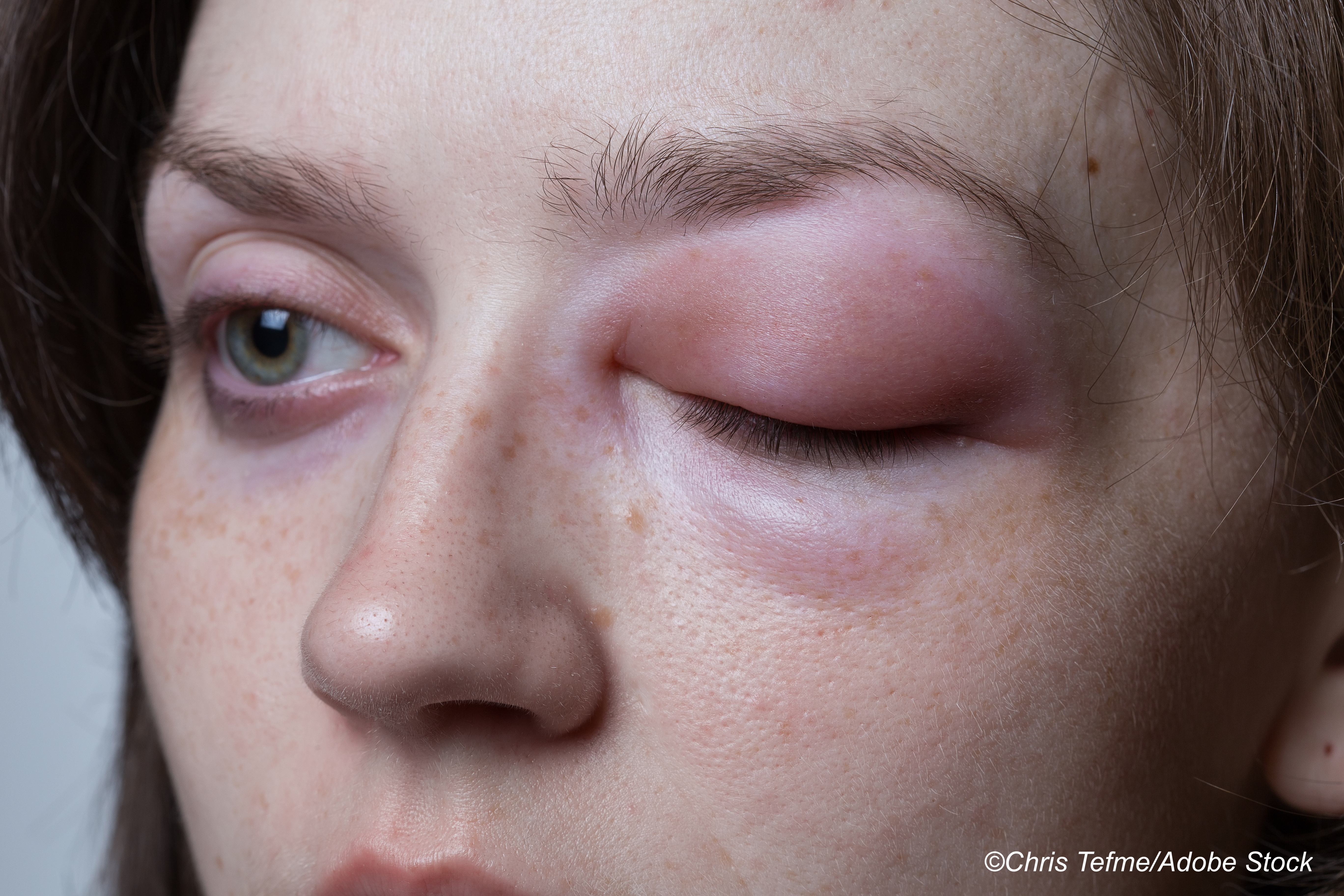
Garadacimab is a first-in-class, fully-human monoclonal IgG4 antibody against activation of factor XII (FXIIa), which initiates the kallikrein-kinin system and leads to the formation of bradykinin, both of which play a key role in hereditary angioedema.
"Hereditary angioedema attacks are caused by spontaneous, uncontrolled activation of the plasma kallikrein–kinin system and overproduction of the vasoactive peptide bradykinin, which increases endothelial permeability and causes subsequent extravasation of fluids into interstitial tissues," explained Craig et al.
Orphan-drug designation for garadacimab as an investigational therapy for hereditary angioedema has been granted by both the FDA and the European Medicines Agency (EMA), and it is also currently being studied for the treatment of other diseases where the inhibition of FXIIa may be important, such as pulmonary fibrosis.
In the VANGUARD trial, Craig and fellow researchers sought to assess the safety and efficacy of once-monthly treatment with subcutaneous garadacimab as prophylaxis for hereditary angioedema. They enrolled 64 patients aged 12 and older with type I or type II hereditary angioedema from Canada, Germany, Hungary, Israel, Japan, the Netherlands, and the U.S.
Most patients were women (59%) and White (86%), and were randomized to garadacimab or placebo for 6 months, stratified by age (≤17 years versus >17 years) and baseline attack rate (1 to <3 attacks/month versus ≥3 attacks/month) in adults. Treatment was comprised of a 400-mg loading dose of subcutaneous garadacimab given as two injections of 200 mg each or placebo in the same volume on day 1, followed by five self- or caregiver-administered monthly doses of garadacimab (200 mg) or placebo.
The primary endpoint of the trial was investigator-assessed time-normalized number of hereditary angioedema attacks per month over the 6-month treatment period.
Over 6 months, patients treated with garadacimab had significantly fewer investigator-confirmed hereditary angioedema attacks per month (mean, 0.27; 95% CI, 0.05-0.49) compared with the placebo group (mean, 2.01; 95% CI, 1.44-2.57; P<0.0001), for a mean percentage difference of –87% (95% CI, –96 to –58; P<0.0001). Garadacimab-treated patients also had a significantly lower median number of hereditary angioedema attacks per month compared with placebo (0 versus 1.35, respectively).
From the first dose of treatment, a full 77% of patients treated with garadacimab had no attacks for at least 72 days, compared with 76% of patients treated with placebo who had no attacks for at least 5 days. Further, over 50% of patients treated with garadacimab remained free of attacks over the 6-month treatment period.
Quality of life was also assessed, and researchers noted, "For patients in the garadacimab group, a clinically meaningful improvement (≥6 points) of the mean [Angioedema Quality-of-Life] AE-QoL questionnaire total score at day 31 was observed, with a 23.7-point reduction from the run-in period. Further improvements were observed at day 182, with a 26.5-point reduction. For patients in the placebo group, there was less than a 6-point mean reduction from the run-in period at any timepoint during the 6-month trial."
The most common treatment-emergent adverse events included upper respiratory tract infections (10% garadacimab versus 8% placebo), nasopharyngitis (8% versus 4%, respectively), and headaches (8% versus 16%).
Researchers also noted that inhibition of activated factor XII was not associated with an increased risk of bleeding or thromboembolic events, with no event occurring in either group.
"Our results support the use of garadacimab as a potential prophylactic therapy for the treatment of hereditary angioedema in adolescents and adults," concluded Craig and colleagues.
The strengths of this study include its strong methodology and design, the inclusion of patients aged 12 years and older, a primary endpoint that was investigator-assessed, and secondary endpoints that assess quality of life, wrote Nicolas Javaud, MD, PhD, of the Université Paris Cité, Urgences, AP-HP, Centre de référence des angiœdèmes à kinines, Hôpital Louis Mourier, Colombes, France, and Delphine Gobert, MD, of Sorbonne Université, Médecine Interne, AP-HP, Centre de référence des angiœdèmes à kinines, Hôpital Saint-Antoine, Paris, France, in their accompanying editorial.
Limitations included the small sample size, short follow-up, and lack of racial and ethnic diversity, they added.
"Therapeutic options for patients with type I and type II hereditary angioedema are rapidly expanding, offering the hope of personalizing long-term prophylaxis. Prophylactic monthly garadacimab significantly reduced the number of hereditary angioedema attacks and could be used as a potential prophylactic therapy for patients with hereditary angioedema," concluded Javaud and Gobert.
Disclosures
This study was funded by CSL Behring.
Craig is a speaker for Pharming, CSL Behring, Takeda, Fresenius Kabi, and Grifols; has received research and consultancy grants from CSL Behring, Takeda, BioCryst, Ionis, Spark, BioMarin, Fresenius Kabi, and Grifols; and is on the medical advisory board for the US Hereditary Angioedema Association, Director of ACARE Angioedema Center at Penn State University, Hershey, and on the Board of Directors for the American Academy of Allergy, Asthma, and Immunology.
Javaud reported no disclosures.
Gobert declared consultancies, speakers’ fees, and travel or accommodation payments by Takeda, Biocryst, Pharming, Kalvista, and CSL Behring.
Sources
Craig TJ, et al "Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicenter, randomized, double-blind, placebo-controlled, phase III trial" Lancet 2023; DOI: 10.1016/S0140-6736(23)00350-1.
Javaud N, Gobert D "Hereditary angioedema: is there a better future for treatment?" Lancet 2023; DOI: 10.1016/S0140-6736(23)00438-5.