JAK Inhibitor Cream Ruxolitinib Appears Safe As Eczema Tx in Young Children
Phase I study findings showed no significant safety signals
04/07/2023
Salynn Boyles, Contributing Writer, BreakingMED™
Kevin Rodowicz, DO, Assistant Professor, St. Luke’s University/Temple University
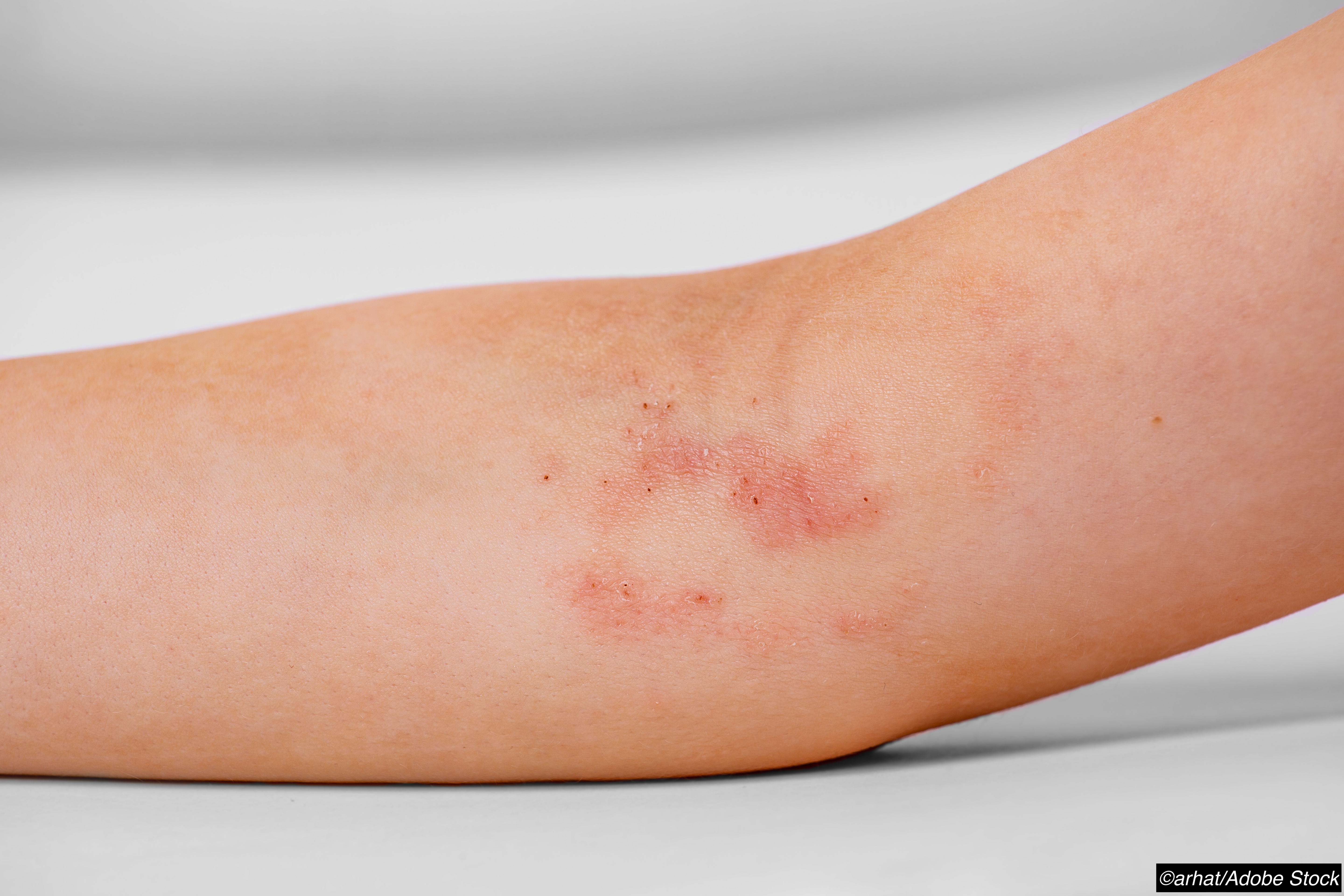
Short-term treatment with the janus kinase (JAK) inhibitor topical cream ruxolitinib was well tolerated at different strengths in children with eczema as young as 2 years of age in a phase I clinical trial.
No impact was reported on blood counts or bone biomarkers during 4 weeks of treatment in the cohort of children and teens, age 2-17 years.
Short-term treatment with the Janus kinase (JAK) inhibitor topical cream ruxolitinib was well tolerated at different strengths in children with eczema as young as 2 years of age in a phase I clinical trial, with no impact reported on blood counts or bone biomarkers.
The FDA-approved the topical version of the JAK1/JAK2 inhibitor in 2021 for short-term use in patients age 12 years and older with atopic dermatitis. The cream received FDA approval last July for the treatment of adolescents and adults with non-segmental vitiligo.
"The plasma concentration of ruxolitinib after topical application was generally low and exhibited a high level of inter-patient variability, consistent with data from previous studies in adults," the researchers wrote. "Ruxolitinib cream may offer benefit in patients who do not respond to topical steroids on the basis of the difference in mechanisms of action between the 2 treatments."
The researchers noted that Janus kinases "mediate the pathogenesis of atopic dermatitis (AD) and pruritus by interfering with the transduction of signals from key proinflammatory cytokines such as interleukin (IL)-4, IL-5, IL-13 and thymic stromal lymphopoietin."
In the pivotal phase III trials TRuE-AD1 and TRuE-AD2, ruxolitinib cream showed anti-inflammatory activity in children 12 years of age and older, and in adults.
"Because AD is substantially more prevalent in children than in adults, and systemic absorption of topical agents may be higher in children owing to greater BSA-to-weight ratios, it is important to examine the safety and tolerability of ruxolitinib cream in pediatric populations," Leung and colleagues wrote.
The researchers conducted the phase I, open-label study at 17 study sites in the United States, enrolling children and adolescents between the ages of 2- and 17-years with AD, an Investigator’s Global Assessment (IGA) score of 2 or more (moderate to severe), and eczema affected surface area of 8% to 20%.
The stepwise design included six age-descending, dosage increasing cohorts with dosages of 0.5%, 0.75%, or 1.5% applied twice a day for 4 weeks.
Forty-four (62%) of the 71 enrolled patients had baseline IGA scores of 3, and the median body surface area affected at baseline was 12.2% (1.7%-20.4%).
"Ruxolitinib cream was well tolerated, with 4 patients (5.6%) experiencing treatment-related adverse events (all grades 1/2)," the researchers wrote. "No clinically meaningful changes in mean chemistry or hematology values were observed, and no consistent pattern of change in bone biomarkers was detected."
Mean plasma ruxolitinib levels within each cohort (range, 23.1-97.9 nM) were below the half-maximal inhibitory concentration for thrombopoietin phosphorylation.
Improvements in exploratory efficacy end points were reported among all cohorts, and across all age groups and formulation strengths and the improvements were maintained or increased by week 4.
"Efficacy results, albeit from a small population assessed in a study of open-label design, were of similar magnitude to those previously observed at week 4 for an adolescent and adult population with AD applying 1.5% ruxolitinib cream in the phase III, double-blind vehicle controlled TRuE-AD trials," the researchers wrote.
"Importantly, similarly to previous studies in adolescents and adults, the use of ruxolitinib cream showed a rapid decrease in pruritus that was sustained to the end of the study. This effect, in addition to providing relief to patients, may be a key to increasing patients’ compliance with therapy."
The researchers cited as a study limitation the small cohort size, which precluded accurate measurement of drug accumulation in younger study participants.
The study also excluded patients with very severe (>20% BSA involvement) eczema, and treatment duration was limited to just 4 weeks, "which does not allow for conclusions about the long-term safety and efficacy of ruxolitinib cream," the researchers wrote.
Disclosures
Funding for this research was provided by Incyte Corporation. Leung reported receiving advisor, investigator and/or speaker fees from Boehringer Ingelheim, Evomune, Incyte Corporation, LEO Pharma, Genentech, Regeneron, and Sanofi Genzyme. Several investigators reported being employed by Incyte Corporation.
Sources
Leung DYM, et al "Safety, pharmacokinetics, and efficacy of ruxolitinib cream in children and adolescents with atopic dermatitis" Ann Allergy Asthma Immunol 2023; DOI: 10.1016/j,anai.2022.12.033.