HER-2 Targeting Tx and Interstitial Lung Disease: Limiting Risk
Experts offer guidance on the management of ILD tied to the HER2-targeting therapy
02/13/2024
Shalmali Pal, Contributing Writer, BreakingMED™
Vandana G. Abramson, MD, Associate Professor of Medicine, Vanderbilt University Medical Center
The adverse event of interstitial lung disease (ILD)/pneumonitis associated with trastuzumab deruxtecan (T-DXd) treatment in multiple solid tumors is generally low grade and easily managed, but providers and patients need to be on board with recommended management strategies.
Recommended avenues for monitoring and managing T-DXd–related ILD/pneumonitis include the "5 S Rules;" the use of imaging studies as well as microbial and serological testing; and/or the "MONITOR-MANAGE-MODIFY strategy."
HER-2/ERBB2-directed therapies have proven their mettle in a range of solid tumors with HER-2 overexpression, including bladder, breast, lung, gynecologic, and gastrointestinal, particularly with the DESTINYGastric trials.
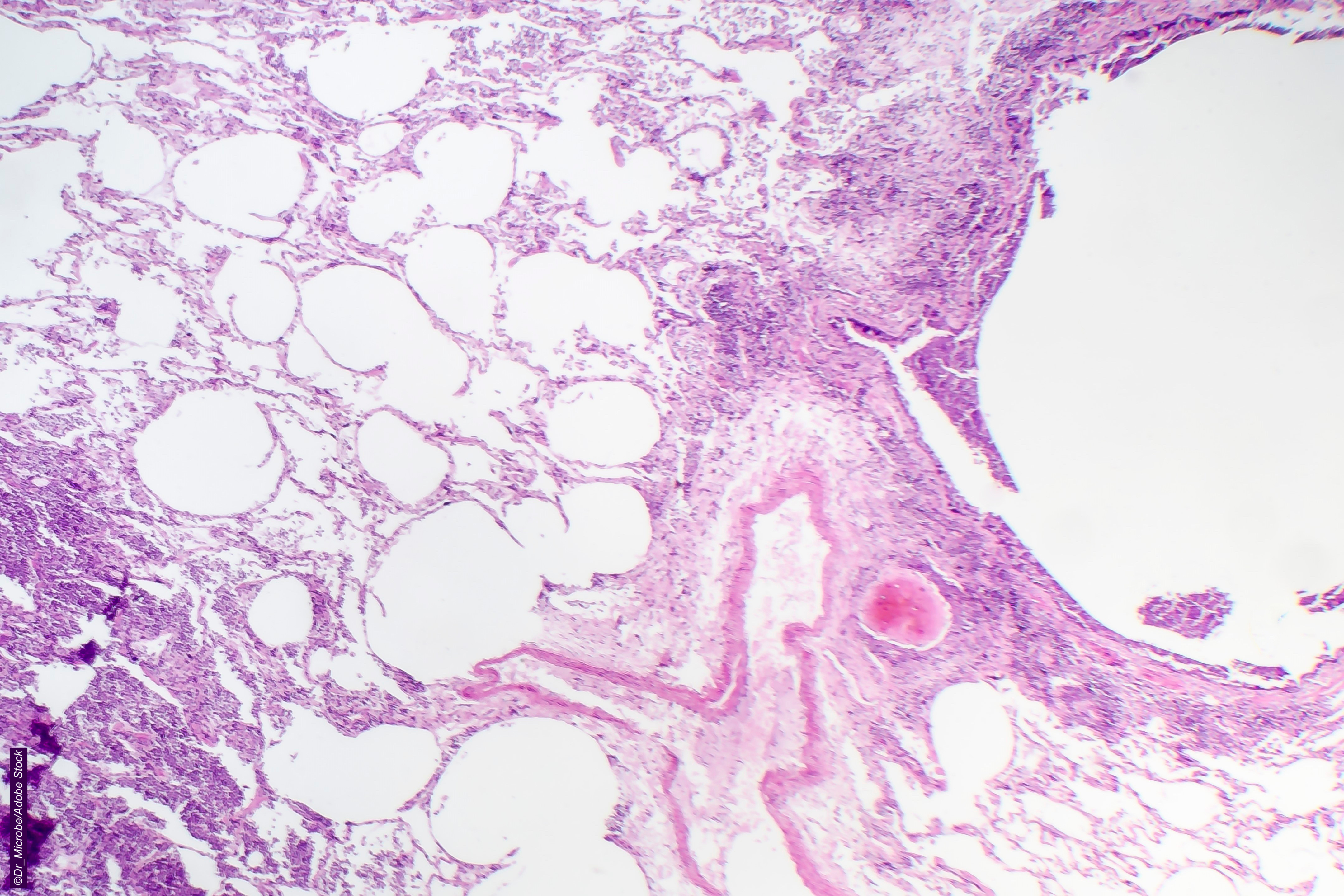
Of course, even the most finely-tuned treatment will come with its fair share of adverse events (AE) but some AEs require a higher level of attention. In the case of HER2-directed therapies, and T-DXd in particular, interstitial lung disease (ILD)/pneumonitis is "one to watch."
While in the majority of cases, ILD is low grade (grade less ≤2) and can be treated effectively," it "may develop to be fatal in some instances," cautioned Sandra M. Swain, MD, of Georgetown Lombardi Comprehensive Cancer Center and MedStar Health in Washington, and co-authors, in a 2022 Cancer Treatment Reviews article. "It is important to increase patient and provider understanding of T-DXd–related ILD/pneumonitis to improve patient outcomes."
ILD on Trial
Swain’s group explained that ILD "is a heterogeneous group of over 200 lung disorders," that [m]anifest as inflammation and/or fibrosis in the lungs…the term pneumonitis is sometimes used because patients can have a preexisting chronic ILD from other conditions, thereby complicating the use of the term ILD for acute drug reactions."
They also noted that in the T-DXd trials, ILD/pneumonitis was broken down into five grades:
- Grade 1: Asymptomatic with radiologic findings only.
- Grade 2: Symptomatic; medical intervention indicated; key activities of daily living (ADL) are limited.
- Grade 3: Severe symptoms; limited self-care ADLs; oxygen indicated.
- Grade 4: Life-threatening respiratory compromise; need for urgent intervention (tracheotomy, intubation).
- Grade 5: Death.
ILD instances in T-DXd trials were on the lower end of the grade scale. For instance, in the phase II DESTINY-PanTumor02 trial, which took all-comers with HER2-overamplified solid tumors (endometrial, cervical, ovarian, bladder, biliary tract, pancreatic, and other), adjudicated drug-related ILD/pneumonitis occurred in about 10% of patients, but the majority of cases were low grade, reported Funda Meric-Bernstam, MD, of the MD Anderson Cancer Center in Houston, and co-authors. They also stressed in the 2023 Journal of Clinical Oncology study that "T-DXd-related ILD/pneumonitis can be safely managed with a multidisciplinary team [MDT]."
In gastric cancer, data from multiple DESTINYgastric trials turned in varying results for ILD. For instance, in DESTINYgastric-01, there were reports that more patients on T-DXd stopped treatment due to AEs including ILD versus those on chemotherapy, but trial authors also stressed that ILD was appropriately managed via active monitoring, dose modification or discontinuation, glucocorticoid therapy, and supportive care. However, in DESTINYgastric-03, there were two deaths tied to ILD/pneumonitis.
Reviews and analyses offered a big-picture view of ILD with HER2-directed therapies. In a 2022 ESMO Open pooled analysis, Charles A. Powell, MD, MBA, of the Icahn School of Medicine at Mount Sinai, in New York City, and co-authors, included nine phase I and II studies of monotherapy T-DXd that described drug-related ILD/pneumonitis.
Their evaluation backed up the assertion that most ILD/pneumonitis instances came in at grades 1 or 2. But Powell’s group said that 87% of patients had their first event within 12 months of their first T-DXd dose, and that adjudicated ILD/pneumonitis onset happened earlier than identified by investigators for more than half the events. Finally, "the median time to the first ILD/pneumonitis event was shorter for grade 5 events compared with the overall median time, suggesting that further investigation of this issue may be warranted," they wrote.
A 2022 Drugs meta-analysis by Misako Nagasaka, MD, of the University of California Irvine, and co-authors, revealed that the highest incidence of ILD/pneumonitis was seen in patients with uterine carcinomatosis (around 27%), followed by non-small cell lung cancer (around 25%), although they acknowledged that the reasons for those findings were not clear and may have been related to a small sample size in their analysis. On the other hand, the lowest incidence of T-DXd-related ILD/pneumonitis was seen in patients with colorectal carcinoma (about 6%) followed by patients with gastric and gastroesophageal junction cancers (about 8%).
What are some take-home messages from these analyses? Nagasaka and co-authors advised that "[g]iven promising efficacy results in multiple settings, the management of potentially fatal adverse events such as ILD would be critical in improving the overall therapeutic index of T-DXd. The underlying mechanism for anti-ERBB2-induced lung damage is yet to be elucidated; however, it is likely to be associated with the cytotoxic agent (payload)," per a 2021 JAMA Oncology review.
Powell and co-authors drew attention to the notion of T-DXd rechallenge, advising that rechallenge may be an option once grade 1 ILD AEs have resolved, but that more research is needed. "[R]echallenge is not recommended for all patients (e.g., patients with grade 2 ILD/pneumonitis). Potential clinical factors of interest for ILD/ pneumonitis may include low SpO [oxygen saturation], lung comorbidities, and renal impairment. Specific risk factors should be confirmed in ongoing and future trials."
ILD Guidance
Swain’s group issued MDT guidance on managing ILD/pneumonitis. Of course, they reiterate that clinicians should be hypervigilant in patients with pre-existing ILD. But there are other risk factors to keep an eye out for, such as age ≥60 years; other pre-existing lung disease; decreased respiratory function; lung radiation exposure; existing renal impairment; and a smoking history.
They also stressed education on ILD/pneumonitis for providers and patients, along with "proactive" patient monitoring, specifically provider education on the interpretation of CT pulmonary findings and other areas; patient education on self-monitoring for new-onset cough or change in exercise tolerance; and close monitoring with an MDT along with visits to the oncologist every six weeks.
In a 2023 JCO Oncology Practice editorial, Sara M. Tolaney, MD, MPH, and Paolo Tarantino MD, both of the Dana-Farber Cancer Institute in Boston, summarized the "5 S Rules" laid out in an accompanying review:
- Screen: Assess baseline risk for ILD before initiating T-DXd; continue to screen during T-DXd treatment. Regular clinical assessments should exclude ILD signs/symptoms.
- Scan: High-resolution chest CT is preferred, starting with baseline scan, and repeated every six to 12 weeks.
- Synergy: Involve the MDT; keep providers and patient well educated on ILD.
- Suspend treatment: If ILD is suspected, T-DXd should always be interrupted; re-started only for asymptomatic, fully resolved ILD.
- Steroids: Corticosteroids are the primary form of ILD treatment, with dosing adapted to toxicity grade.
"[O]ptimizing the detection and management of ILD will be critical in the setting of the current development of T-DXd in the curative space, where the high antitumor activity of T-DXd could have a major therapeutic impact, but also where an extremely cautious balance of risks and benefits is warranted," stated Tolaney and Tarantino.
Salvatore Siena, MD, of the Grande Ospedale Metropolitano Niguarda in Milan, and co-authors, put together an expert opinion on the management of drug-induced ILD (DIILD), writing in a 2023 ESMO Open article that "[b]ecause the clinical and radiological signs of DIILD are often similar to those of pneumonias or interstitial lung diseases, differential diagnosis is important, including microbial and serological testing to exclude or confirm infectious causes." Non-imaging tests should use a "blood sample…for a complete blood count with differential, and tests for liver and kidney function and inflammatory markers, such as erythrocyte sedimentation rate, C-reactive protein and lactate dehydrogenase. In the case of circulatory shock, a procalcitonin assay should also be carried out," they advised.
In 2023, Rebecca Dent, MD, of the National Cancer Centre Singapore, and co-authors formed an Asia-Pacific multi-disciplinary expert panel, and pointed out that the management of ILD/pneumonitis linked with T-DXd "may need to account for regional considerations, such as reimbursement and practicality." For instance, they recommended in their 2023 Drug Safety article that in the Asia-Pacific region, if the schedule of a CT scan every six weeks is not feasible, then supplemental testing with a six-minute walk test or one-minute sit-to-stand tests at baseline and follow-up are options.
Finally, the T-DXd warning label calls for the "MONITOR-MANAGE-MODIFY strategy" in patients with suspected ILD, including higher-frequency monitoring for those with moderate renal impairment; following even low-grade cases of ILD until resolution and through treatment discontinuation; and avoiding the re-escalation of T-DXd after dose reduction.
Disclosures
The DESTINY-PanTumor02 and DESTINYgastric trials are supported by AstraZeneca and Daiichi Sankyo. Some co-authors are AstraZeneca or Daiichi Sankyo employees.
Meric-Bernstam and co-authors reported relationships with, and/or support from, multiple entities including AstraZeneca and Daiichi Sankyo.
Swain and co-authors reported support from ArticulateScience/AstraZeneca. Swain reported relationships with, and/or support from, Breast Cancer Research Foundation, Genentech/Roche, Kailos Genetics, AstraZeneca, Daiichi Sankyo, Molecular Templates, Silverback Therapeutics, Eli Lilly, Merck, Natera, Exact Sciences, Athenex, Biotheranostics, the Beijing Medical Foundation, Caris, ASCO Conquer Cancer Foundation, and The National Surgical Adjuvant Breast and Bowel Project Foundation. Co-authors reported relationships with, and/or support from, multiple entities including AstraZeneca and Daiichi Sankyo.
The analysis by Powell’s group was funded by AstraZeneca and Daiichi Sankyo. Some co-authors are employees of Daiichi Sankyo. Powell and co-authors reported support from ArticulateScience/AstraZeneca. Powell reported relationships with Daiichi Sankyo, AstraZeneca, and Voluntis. Co-authors reported relationships with, and/or support from, multiple entities including AstraZeneca and Daiichi Sankyo.
Nagasaka reported relationships with, and/or support from, AstraZeneca, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Janssen, Pizer, Eli Lilly, Genentech, Caris Life Sciences, Blueprint Medicines, and AnHeart Therapeutics.
Siena and co-authors reported support from Daiichi Sankyo S.p.A. and AstraZeneca S.p.A. Siena reported relationships with, and/or support from, Agenus, AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol Myers Squibb, CheckMab, Daiichi Sankyo, Merck Serono, and Seagen. Co-authors reported relationships with, and/or support from, multiple entities including AstraZeneca and Daiichi Sankyo.
Sources
Swain S, et al "Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis — Focus on proactive monitoring, diagnosis, and management" Cancer Treat Rev 2022. DOI: 10.1016/j.ctrv.2022.102378.
Powell CA, et al "Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies" ESMO Open 2022. DOI: 10.1016/j.esmoop.2022.100554.
Abuhelwa Z, et al "Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-positive advanced solid malignancies: A systematic review" Drugs 2022;82:979-987. DOI: 10.1007/s40265-022-01736-w.
Conte P, et al "Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment" ESMO Open 2022. DOI: 10.1016/j.esmoop.2022.100404.