U.K. Study Doubles Down on TAVI vs SAVR Equipoise
No difference in all cause mortality at 1 year
05/17/2022
Peggy Peck, Editor-in-Chief, BreakingMED™
Vandana G. Abramson, MD, Associate Professor of Medicine, Vanderbilt University Medical Center
In a pragmatic design, randomized clinical trial of aortic stenosis patients considered at moderate high risk for surgery, TAVI was non-inferior to surgical valve implant.
Be aware that the findings from this U.K. study confirm previous trials that found TAVI a safe and effective alternative to surgical aortic valve implant.
A trial conducted at 34 sites within the United Kingdom confirmed that patients age 70 or older with symptomatic aortic stenosis who have a "moderately increased surgical risk" will do as well with a transcatheter aortic valve implant (TAVI) as they will with surgical implant.
The issue of TAVI—also known as TAVR—versus surgical aortic valve replacement (SAVR) has been debated and studied for almost a dozen years since the pivotal initial results of PARTNER I found that patients who were too frail to undergo the rigors of surgery could benefit by having a new aortic valve implanted using catheter-based technology. Those researchers quickly followed that report with results from the second part of the PARTNER I trial, comparing TAVI to surgery. The patients in that second cohort were also very high risk, but well enough to withstand surgery and, in that comparison, the one-year mortality was 2.6% lower with TAVI (P=0.001 for noninferiority).
The PARTNER investigators continued to throw a wider net for TAVI, eventually reporting the efficacy of TAVI for intermediate to moderate risk aortic patients.
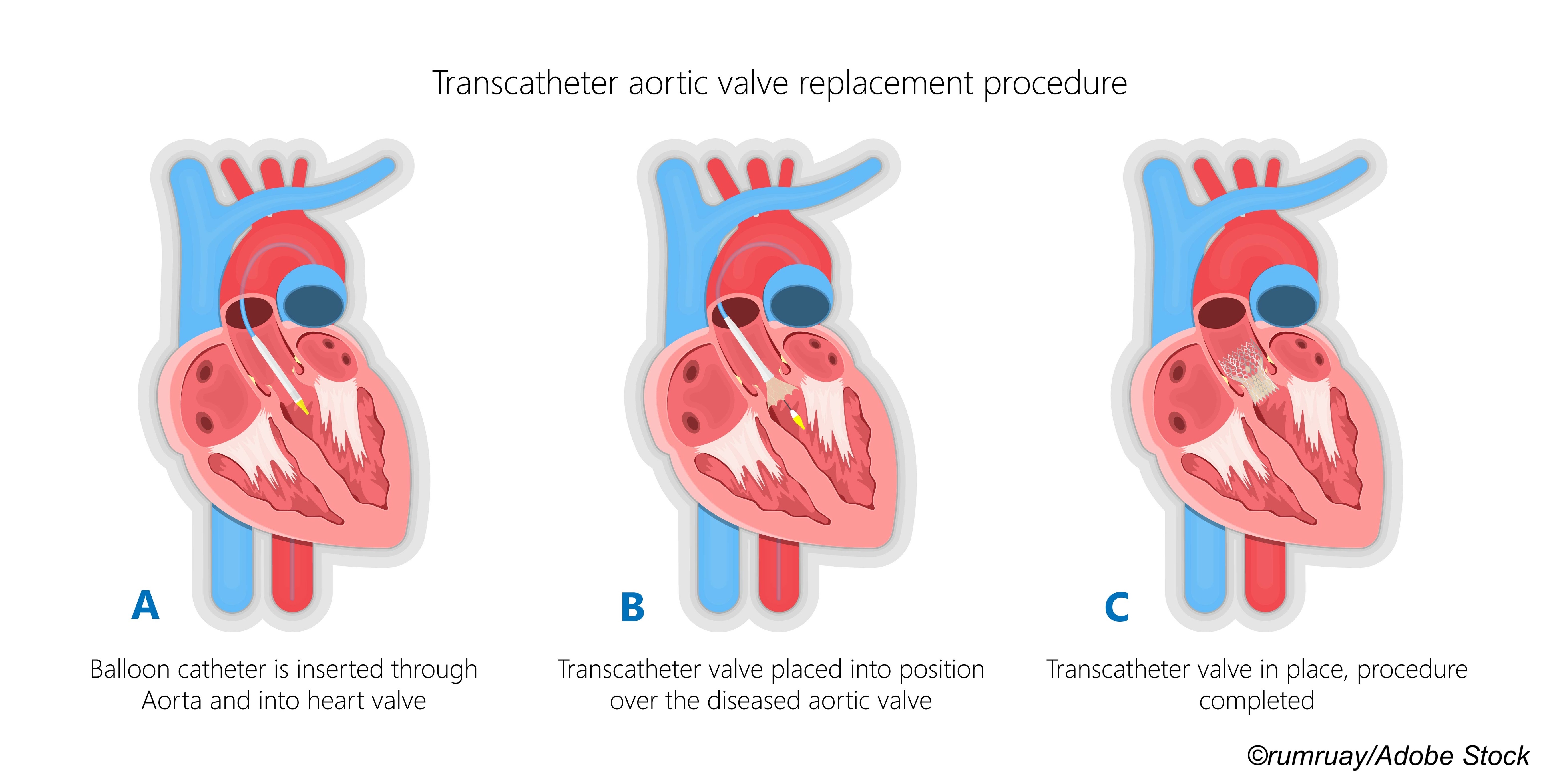
In this latest TAVI versus surgery trial, the U.K. TAVI Trial investigators enrolled 913 patients (median age 81, 46% women). They randomized 458 to TAVI and 455 to surgery. The primary outcome was all-cause mortality at 1 year.
"At 1 year, there were 21 deaths (4.6%) in the TAVI group and 30 deaths (6.6%) in the surgery group, with an adjusted absolute risk difference of −2.0% (1-sided 97.5% CI, −∞ to 1.2%; P<.001 for noninferiority)," wrote William D. Toff, MD, of the University of Leicester, in Leicester, England, and co-investigators with the U.K. TAVI Trial in JAMA.
And when Toff et al assessed a long list of secondary outcome measures that included length of stay, bleeding, vascular complications, need for pacemaker implantation, and aortic regurgitation, they found that 24 of 30 measures showed no significant differences at 1 year.
Also among the findings:
- Hospital length of stay (LOS) was shorter with TAVI—3 versus 8 days (P=0.11).
- Rate of major bleeding events was lower—7.2% versus 20.2% (P<0.001).
- Myocardial infarction events were slightly higher—6 versus 5 (P=0.81).
- Prevalence of any reintervention was lower—30 versus 37 (P=0.31).
- Prevalence of cardiovascular death was lower—13 versus 15 (P=0.69).
They did, however, find more vascular complications (10.3% versus 2.4%; P <0.001) and more pacemaker implants (14.2% versus 7.3%; P <0.001) with TAVI.
The investigators noted that unlike previous trials, which were funded by industry, the current trial was "pragmatic, publicly funded, and designed to compare a TAVI strategy using any valve type and access route versus a conventional surgery strategy in a broad range of patients."
The findings are not surprising given the plethora of TAVI or TAVR studies that preceded them, including the 5-year outcomes results from the PARTNER 2 investigators, who wrote in The New England Journal of Medicine that there was no "significant difference in the incidence of death or disabling stroke at 5 years after TAVR as compared with surgical aortic-valve replacement."
In an editorial published with the U.K. trial results, Catherine M. Otto, MD, PhD, of the University of Washington School of Medicine in Seattle, and Jae-Kwan Song, MD, of the Univeristy of Ulsan College of Medicine in Seoul, South Korea, raised the issue of durability for TAVI bioprostheses, suggesting that it is likely that with time there may be evidence of "…leaflet calcification and valve degeneration, although the time course remains uncertain; thus, longer-term outcome data for patients undergoing TAVI are needed. To date, this has been a challenge because most patients undergoing TAVI, as in the current study, typically are older than 70 years of age or even aged 80 years leading to limited follow-up duration and a marked survival bias. All these factors limit extrapolation of outcome data on TAVI to younger patients."
But the most recent outcomes data reported—findings from the SURTAVI Intermediate Risk Trial reported in early April at the American College of Cardiology—suggest that TAVI valves may be more durable than surgically implanted valves. In a pooled analysis that examined the 5-year incidence, outcomes, and predictors of hemodynamic structural valve deterioration (SVD) among patients who underwent replacement using a trans-aortic valve implant (TAVI) versus surgical implant, researchers reported a "significantly lower rate of SVD with TAVI versus surgery—4.38% versus 2.57%, (P=0.0095 [Fine-Gray P value])," said Michael J. Reardon, MD, professor of cardiothoracic surgery and Allison Family Distinguished chair of cardiovascular research at Houston Methodist DeBakey Heart and Vascular Center.
In an interview at the time of his ACC presentation, BreakingMED asked Reardon to put the SURTAVI findings in clinical perspective. "I don’t think this indicates need for a change in guidelines, but it certainly will change the conversation within the heart team, and it will change my conversation with patients. I can’t say surgery is better, will give you a longer lasting valve," Reardon explained.
Megan Coyleweight, MD, vice-chief of cardiology and director of the structural heart unit at Erlanger Heart and Lung Institute in Chattanooga, Tennessee, who moderated an ACC session on aortic stenosis, said the SURTAVI results were especially important, "because this is not what we’ve been telling patients, which is particularly important as we see young patients seeking AV replacement—patients who expect to live longer. We don’t want to see them coming back again and again for replacements."
Cardiothoracic surgeon James B. McClurken, MD, of Doylestown Health Cardiothoracic and Vascular Surgery outside of Philadelphia agreed with Coyleweight, noting that the belief that surgically implanted valves were more durable was based on "the fact that we’ve been using them longer."
Otto and Song concluded their editorial with this important point: "The best valve for each patient is their own valve; the goal should be to ensure that their own valve functions normally. Treatment of aortic stenosis with only surgical aortic valve replacement or TAVI is analogous to treating coronary artery disease only with coronary artery bypass graft surgery or percutaneous coronary intervention. The effects of primary and secondary prevention of coronary artery disease with lifestyle behaviors and medications, including not smoking, a heart-healthy diet, regular exercise, maintaining a normal bodyweight, treatment of hypertension, hyperlipidemia, and diabetes are far more important than mechanical interventions to relieve obstruction with end-stage disease."
Disclosures
The study was funded by the National Institute for Health Research Health Technology Assessment Programme. Additional support was provided by the National Institute for Health Research Clinical Research Network. Excess treatment costs were paid by the NHS England in England, by the National Institute for Social Care and Health Research in Wales, and by the Chief Scientist Office and NHS Research Scotland in Scotland.
All authors reported receiving grant funding from the National Institute for Health Research that was awarded to their institution during the conduct of the study.
Otto and Song had no disclosures.
Sources
Toff WD and the UK TAVI Trial Investigators "Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: A randomized clinical trial" JAMA 2022; 327(19): 1875-1887. DOI: 10.1001/jama.2022.5776.
Otto CM, Song JK "Treatment of aortic stenosis with transcatheter aortic valve" JAMA 2022; 327(19): 1870-1871.